
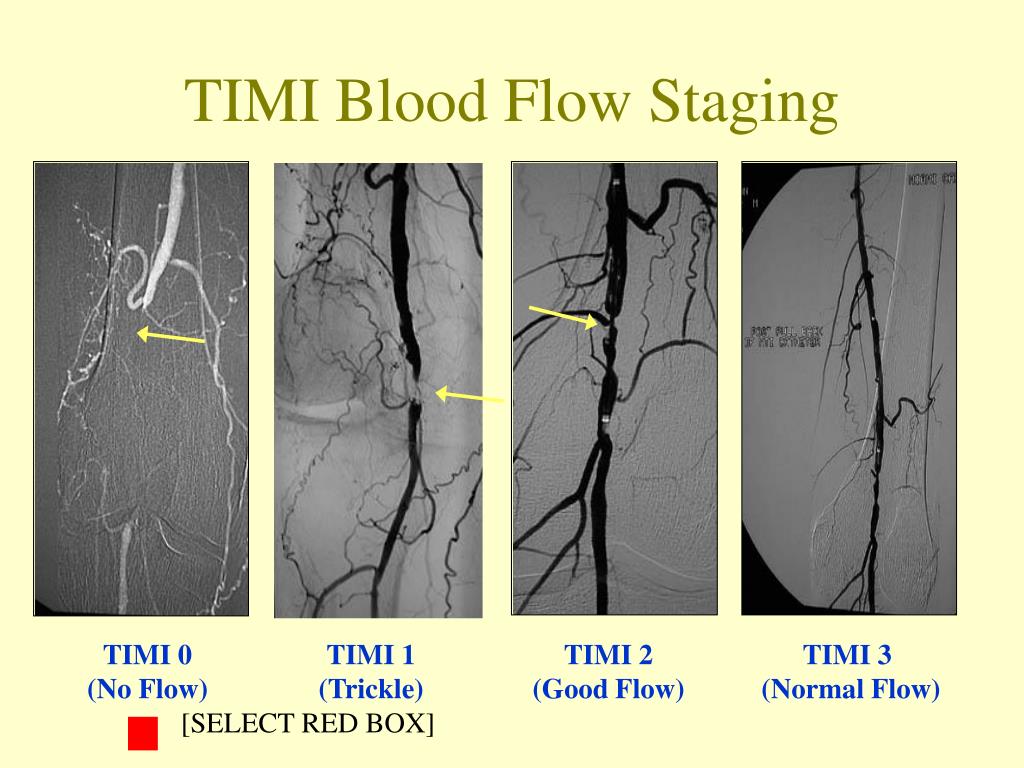
Cequier reports receiving consulting/lectures fees from AstraZeneca, Abbott Vascular, Medtronic, Boston Scientific, Biotronic, Ferrer, and Daiichi Sankyo, and grant support from Abbott Vascular, Medtronic, and Spanish Society of Cardiology. Bolognese reports receiving consulting and lecture fees and fees for board membership from Daiichi Sankyo, Eli Lilly, Menarini, AbbottLaboratories, AstraZeneca, and Iroko Cardio International. Vicaut reports receiving fees for serving on a data and safety monitoring board at the European Cardiovascular Research Center, consulting fees from Abbott Laboratories, Bristol-Myers Squibb, Celgene, Fresenius, LFB, Eli Lilly, Medtronic, Pfizer, and Sanofi-Aventis, lecture fees from Novartis, and grant support from Boehringer Ingelheim and Sanofi-Aventis. Zeymer reports personal fees from AstraZeneca, during the conduct of the study personal fees from AstraZeneca, grants and personal fees from Daiichi Sankyo, Eli Lilly, personal fees from Bayer Healthcare, The Medicines Company, grants and personal fees from Sanofi, Novartis, personal fees from Boehringer Ingelheim, MSD, outside the submitted work. Bauer has received speaker fees from AstraZeneca. In this post-hoc analysis, preprocedural TIMI flow was not independently associated with a higher rate of adverse ischemic events.ĭr. Among these patients, those with prehospital administration of ticagrelor were less often affected (0.3% vs. However, definite stent thrombosis occurred only in patients with initial TIMI flow 0/1 (1.0%). After adjustment, preprocedural TIMI flow <3 (versus 3) was not an independent predictor of major adverse ischemic events within 30 days (odds ratio 1.89, 95% confidence interval 0.74–4.85). At 30 days, the composite ischemic endpoint (5.5, 2.9, and 2.1%, p < .05) and all-cause death (3.0, 1.4, and 2.1%, p = .30) were highest in patients with TIMI flow 0/1. RESULTSįrom a total of 1,680 patients, 1,113 had TIMI 0/1, 279 TIMI 2, and 288 TIMI 3 flow before primary PCI. For this analysis, patients were divided into three groups according to the preprocedural TIMI flow grade of the infarct vessel: TIMI 0/1, TIMI 2, and TIMI 3. The ATLANTIC study compared prehospital versus inhospital treatment with ticagrelor in patients with acute STEMI. However, it is unclear whether the same is true in patients with ongoing STEMI of less than 6 hr duration, rapid reperfusion, and modern guideline-adherent therapy. Previous studies have shown that the TIMI flow 0/1 prior to primary percutaneous coronary intervention (PCI) is associated with a poor clinical outcome. This study sought to analyze the impact of the preprocedural thrombolysis in myocardial infarction (TIMI) flow on clinical outcome in patients with ST-elevation myocardial infarction (STEMI).


 0 kommentar(er)
0 kommentar(er)
